Cold war against hydrates
Researchers are on the brink of a breakthrough. They want to prove that oil can be transported from the bottom of the ocean in bare steel pipes – without insulation or warming devices. This smells of money.

Researcher Marita Wolden ensures that the pressure in the research loop is stable.
Photo: Rune Petter Ness
The luminous green line on the floor in front of me shows the way out. I put on my safety glasses well, aware that SINTEF researcher Roar Larsen has given a clear message: “In the event of fire or an explosion, follow the line.
Then we will meet up for a roll call behind the workmen’s hut down the hill”.
“Is everything OK?”
“Yes, OK”.
We are then ready to enter the closed laboratory world at SINTEF’s Multiphase Flow Laboratory just out of Trondheim. While here, we will take a closer look at a test that, if successful, can save the oil industry millions of dollars.
Explosive item
The reason for the safety procedures is that the researchers are experimenting with one of nature’s most explosive and inflammable compounds – gas hydrates. The hydrates are an expensive problem for oil companies. They often halt production and, in addition, are extremely inflammable.
Complex and expensive solutions are required when oil and gas are transported in the same pipeline along the sea floor. This joint transportation (multiphase flow) makes it possible to expand oil fields without platforms – fields that would not otherwise have been profitable.
“The problem is that under low temperatures and high pressure, water and natural gas form hydrates or ice-like lumps that clog oil pipelines”, says Roar.
“Clogged pipelines result in a complete stop in production. In addition, the hydrates constitute a huge risk when they are removed because they often require heating, something that can lead to a large increase in pressure and subsequently an increased risk of explosion”, he explains, while leading us into the cold laboratory. The temperature inside is 4 degrees Celsius, like on the sea floor.
Ice-cold alternative
The stubborn clumps are formed under high pressure and low temperature, as in oil production or at extreme sea depths. The oil industry currently utilises several methods to control the formation of hydrates. All involve maintaining warmth in the transport pipeline or adding a chemical cocktail that reduces the freezing temperature of the oil. This costs a huge amount of money.

The hydrates are formed at the water/oil interface. Droplets of water in the oil phase become enveloped by a thin layer of gas hydrates.
Now Roar and his colleagues believe they have a cold and cunning alternative to control the hydrates, which is known as Cold Flow technology. In theory, the solution is ready. It is now being tested out in the laboratory we are standing in. If the test is successful, in a few years oil will be transported up from the deep in completely ordinary steel pipes without insulation and other bits and pieces. And the energy-rich gas hydrates found in crude oil will be able to be separated as a dry powder!
As one cubic metre of gas hydrates can contain 170 cubic metres of gas, the laboratory is constructed as a closed atmosphere. For logical reasons, every item that could cause a spark is kept out. As mobile phones contain components with high voltage, they are strictly prohibited here. The photographer’s digital camera just makes it inside.
A 50 metre long and twisted formation of pipes, pumps and ventilators stretches out before us. It is managed by researcher Marita Wolden, who is wearing orange fire-retardant, wool-lined overalls and safety glasses.
Crude oil is circulating in the pipes and somewhere in there are gas hydrates – hopefully in a controlled and safe state.
Despite all the safety procedures, senior researchers Roar Larsen and Are Lund are rather relaxed. They have spent many hours in this laboratory. In addition, they have comprehensive knowledge on hydrates and Trondheim’s researchers are among the world’s leaders in this field.
The two men have been working on the idea, which is now being tested, for the past six years. So far all the calculations and research indicate that the theory is up to the mark also in practice. Even British Petroleum has been knocking on the door of the Trondheim laboratory. The results they saw were so interesting that BP has now become a co-owner of the project.
From sticky mass for powder
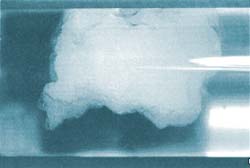
The hydrate-covered water droplets break up, and the water which is released acts as a sort of glue between the hydrate particles. These form sticky clumps like wet snow, which are capable of blocking pipelines.
Roar clarifies the background to the research: “We have studied gas hydrates since the mid 1980s. One of the things we have learned is that during their ‘lifetimes’ hydrates go through three phases. Firstly, they behave like loose, wet snow. After a while they become sticky and form clumps that are like big sticky snowballs. These are the clumps that clog the pipes.
The reason is that during this phase the hydrates contain water that is still not actually part of the hydrate. But if the hydrates have the opportunity to further develop, they end up as dry powder. In this phase, all of the water transforms into pure gas hydrates that float easily and problem-free together with the crude oil”.
“What we needed, therefore, was a procedure to make the gas hydrates develop from the first wet snow phase into the dry hydrates without going through the dangerous phase where they were sticky and slushy”, says chemist Are Lund, who was then responsible for the hydrate tests.
Seed in the deep
After a while Are discovered that it was easier to get lumpy hydrates to become powder than the reverse. Every time they added water to the dry powder to observe how a hydrate plug occurs, more dry hydrates formed instead.
The hydrates still looked like they changed to dry hydrates if they met other dry hydrates at low temperatures.
“The find gave us the idea that is now being tested in the laboratory: If we add dry hydrates as a sort of seed particle in the oil, the sticky hydrates still change to dry hydrates – totally by themselves”, clarifies Are, adding that this technology is now patented.
Behind the glass wall, Marita keeps the pressure in the pipe loop stable. The oil is still circulating in the pipes. The aim of the test is to get it to come to a standstill by saturating the oil with dry hydrates.
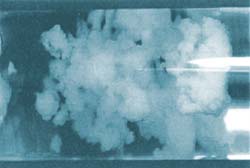
As the water at the surface of the clumps is gradually converted to gas hydrates, the clumps dry out and begin to resemble coral; they are no longer sticky.
“By adding water to form hydrates, we find out how much dry hydrate the oil tolerates before it plugs the pipe. The more it tolerates the better”, says Marita. “So far it is looking good. The oil has already managed to absorb more water than we thought was possible at the outset”.
The plant she is testing is a mini version of the patented solution.
Soon to the sea bottom
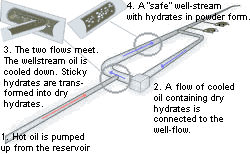
The technology will be far simpler in the sea, says Roar. He explains: “When the warm oil is pumped up from the reservoir, the flow from the well is connected to a flow pipe that mixes cool oil with dry hydrates.
When the two oil flows meet each other, the oil in the warm well flow cools rapidly. Normally the cooling takes a long time, so the oil will form hydrates a long way out in the pipe system.
Now the researchers have control over the cooling and know precisely where in the pipeline the hydrate formation begins. Because dry hydrates are added to the cold oil flow, the sticky hydrates convert to the dry phase after a short time. In this situation they are totally safe and can be transported without danger. The process is automated in that the dry hydrates that are used as seed come from the same pipeline, just a bit further out in the process.
“In this way, we can start a sort of chain reaction. A field often consists of several wells. By connecting a pipe loop containing cooled oil and dry hydrates to the first in a series of wells, the crude oil from several wells can safely be transported up with hydrates that travel in powder form”.
Our man in London
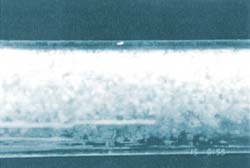
The brittle structure gradually breaks down into small clumps, ending up as a dry hydrate powder which flows easily through the pipeline.
After some attempts, I make telephone contact with BP’s contact person, Dr Carl Argo in London. He says that BP believes strongly in the Cold Flow technology, which he describes as an exciting solution, both technically and commercially.
“The most important advantage with this technology is that expenses for well and satellite hook-ups will be considerably lower. In
addition, it will make it possible to lay even
longer lengths of pipeline than is possible today”, he says. “We want to carry out field research within the next three years and the aim is to commercialise the technology within four years”.
If Cold Flow is successful, BP hopes to license the technology to other companies, he remarks to Gemini. In addition to BP, the project has received important funding from the Research Council of Norway.
Footnote:
The tests were concluded two days later. The researchers found what they were looking for, namely the saturation point for how much dry hydrate oil can contain before transportation stops. That means that researchers will soon be ready for the next big step: field research in deep water in some of the world’s most important oil production areas.
Text: CHRISTINA B. WINGE