Stealth medicine
Using nanocapsules containing cancer drugs, researchers have succeeded in attacking tumours with surgical precision. One of the ways to manufacture such capsules is with minute droplets of super glue.
This new means of delivering drugs has already been successfully tested on mice and rats and, according to researchers at SINTEF, could open the way for entirely new treatments of brain diseases such as Alzheimer’s and Parkinson’s.
SINTEF researchers have developed a new nanocapsule that exploits the tumour’s Achilles heel – poor quality blood vessels which develop as a result of very rapid growth.
“This makes it easier for us to penetrate the vessels and deliver the drugs directly into the tumour”, says researcher Ýrr Mørch, a nanomedicine specialist at SINTEF.
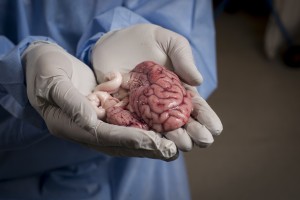
This pig brain will provide researchers with cells that are crucial in helping to get the medicine across the blood-brain barrier. Photo: Geir Otto Johansen.
Nanogas explosions
The nanocapsules are transported in the bloodstream with the help of gas bubbles, and secrete the cancer drug when they arrive at the targeted tumour. The bubbles also provide the drugs with an extra push on encountering the tumour’s blood vessels.
“We intend to use ultrasound to burst the bubbles when the drug reaches the tumour”, says Mørch. “Ultrasound generates a minute pressure wave and increases the tissue temperature. This makes it easier for the nanoparticles to penetrate the blood vessels in the tumour, where they then disintegrate, release their cytotoxins, and kill the cancer cells “, she says.
“Since the gas bubbles also act as a contrast medium, we can use ultrasound to actually observe the drug treatment in action. A further benefit of this method is that the drug can be made to recognise the tumour”, says Mørch.

Gas bubbles carry nano capsules into the bloodstream, where the capsules can secrete cancer medication once they reach the tumour that needs to be treated, explains researcher Ýrr Mørch. The nano-capsules are produced in this reactor. Photo: Christina Benjaminsen.
“The capsules can contain target-seeking molecules which draw them to the tumour”, says Mørch. “This has two useful applications. The first is that the capsules can warn us of the presence of a tumour, which promotes quicker diagnosis. The second is that the tumour in fact attracts the drug to it, making treatment more effective”, she says.
More potent and fewer side-effects
As well as ensuring that a greater proportion of the drug reaches the tumour, the in-built highly localised delivery of the drug in this form of treatment results in fewer side-effects for the patient. Only diseased tissue is treated.
Literature studies show that localised drug delivery means that tumours are exposed to much higher doses than is the case with traditional cytotoxin treatments.
“Under existing treatments, less than one tenth of a per cent of the drug actually reaches the tumour”, explains Mørch. “The rest ends up in the liver, kidneys and other organs, which makes the drug of little use and often leads to side-effects. On the other hand, literature studies show that when we use targeted treatments, up to between 5 and 6 percent of the drug reaches the tumour, which greatly reduces its harmful effects. This makes it a safer form of treatment”, says Mørch, who adds that the current objective is to achieve a “ten per cent” effect using the targeted treatment approach.
This opens the way for the use of super-effective cancer drugs which are not currently on the market because they produce too many side-effects.
“In simple terms, this will allow us to administer lower levels of drugs to patients and at the same time treat the tumour in a more targeted way”, says Mørch.
Stealth particles
One of the challenges researchers are working on is to camouflage the capsules to prevent the patient’s immune system from attacking and destroying the minute drug packages before they reach their target.
“The body is very adept at recognising foreign objects”, says Mørch. “So one of our tasks is to develop an approach which prevents the body from discovering them”, she says.
From "dynabeads" to ultrasound
Researchers here at SINTEF have more than 30 years experience in the development of particles used in various biochemical and medical applications. The best known of these are the so-called "dynabeads" (or Ugelstadkulene), developed as early as 1978. The beads can recognise specific cells and bacteria and are used, among other things, in applications where there is a need to extract specific types of cell, such as from a blood sample or in connection with cancer treatments.
"When drug delivery using nanoparticles really took off between 10 and 15 years ago, it was natural that SINTEF should show an interest in this field", says Ruth Schmid, who is SINTEF's Marketing Director for this field. "Our knowledge of particles was transferred and expanded to include particles and capsules on a much smaller scale. Today, SINTEF employs specialists in particle design and nanomedicine – so we are well equipped to develop some exciting technologies", she says.
In the last three to four years, SINTEF researchers have also been looking beyond their own field, and this is why the new nanocapsules are so effective.
"We have combined the ultrasound expertise of SINTEF and NTNU, and the new treatment method could not have been developed without this cross-institutional collaboration", says Schmid.
Many different groups are working on projects involving drug delivery using nanoparticles. What is special about this project is that the particles are manufactured in a single step and then attached to gas bubbles to achieve more effective targeting, combined diagnostics (imaging) and therapy.
“This delays the body’s ability to identify the particles, and buys us the time we need to deliver them to the tumour”, says Mørch. She adds that the approach has been named “stealth technology” after comparisons with the advanced fighter planes which avoid detection by radar partly because of treatment with a special surface coating.

Colour is added to the nanocapsules, so that researchers can easily see if they are working as they should when they reach the tumor. Photo: Christina Benjaminsen .
Inexpensive, high volume manufacture
The special particles can be manufactured in a single step which means that production is relatively straightforward, and at the same time highly cost-effective.
“In simple terms, we make nanodroplets of glue which are then mixed with the drug”, says Mørch. “This is the same adhesive as we use to bond wounds. The droplets are then hardened by adding a hardener. The glue is gradually broken down in the body and the drug released”, she explains.
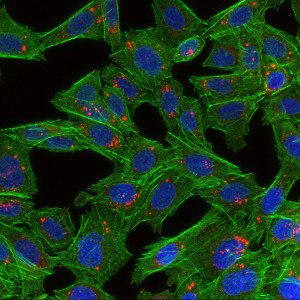
The picture shows cells that have taken up nanoparticles. The cells are green, the cell nuclei are blue and the nanoparticles are red. Photo: Habib Baghirov, NTNU.
The nanoparticles themselves are manufactured in a reactor. Air and proteins are then mixed in with the solution to create the air bubbles which attach themselves to the nanoparticles and stabilized them.
Hope for brain disease treatments
The special technology which exploits the particles’ ability to stabilize gas bubbles may be highly significant for the future treatment of brain diseases such as Alzheimer’s and Parkinson’s.
“We know that it’s difficult to deliver drugs to the brain because it is surrounded by a very effective barrier which keeps the bloodstream and spinal fluid separate. This barrier is designed to protect the brain as far as possible from harmful substances in the blood”, she says. “This is why we have now launched a trial together with physicists at NTNU. Our aim is to find out what intensity of ultrasound we need to get the gas bubbles to penetrate the barrier protecting our brains. If we succeed, it will then be possible to deliver drugs into a part of the body which we currently cannot reach”, says Mørch.
What next?
Much of the research is taken up with investigating what happens to the particles after they have disintegrated and “done their job”.
“The particles will disappear, but some of their components will remain in the body”, says Mørch. “We know that these may be toxic in high concentrations, so it is essential for us to find out how they affect both people and the environment after treatments. The benefits must be greater than the drawbacks”, she stresses.
Long road ahead
The developers are currently talking to SINTEF’s commercialisation company SINVENT in order to secure the rights to the particle manufacturing technology, and as a prelude to opening dialogue with the industry and potential investors.
SINTEF Group Initiative "Medical ACTION"
Since 2006, SINTEF has completed a series of Group-wide initiatives in selected research fields. These Group projects are rooted in our strategy aimed at investing our own funds in expertise development. Medical technology and nanomedicine have been identified by SINTEF as strategic areas with major growth potential. For this reason an internal strategic investment initiative was launched involving three of the SINTEF institutes; SINTEF Materials and Chemistry, SINTEF Technology and Society, and SINTEF ICT. These are currently combining their disciplines to develop new skills and technology platforms. The aim of this initiative is to develop SINTEF into a preferred partner in major international projects addressing certain medical technology fields such as in vitro diagnostics and in vivo imaging diagnostics and treatment. This Group-wide initiative extends from 2011 to 2014, and operates with an annual budget of NOK 6 million. Marketing Director Ruth Schmid acts as Project Manager.
“We are currently conducting pre-clinical studies on small animals”, she says. “The next stage will be to carry out tests on larger animals – probably pigs – and then we will have to demonstrate that the method is safe. If we succeed, we will be ready to persuade the pharmaceutical industry of the usefulness and cost-effectiveness of our approach”, says Mørch.
“We have great faith in this because the entire manufacturing process is completed in one step, facilitating rapid and high-volume production. Manufacturing on a large scale is highly cost-effective. However, getting a new drug onto the market is a marathon, not a sprint”, emphasises Mørch.
The project is being funded by the Research Council of Norway, the Helse Midt-Norge Health Trust and SINTEF.