Norwegian family’s medical mystery solved
Back in the 1970s, a Norwegian family was found to have abnormally high red blood cell counts. Thirty-five years later, researchers succeeded in solving the mystery, thanks to new analytical methods and the latest developments in genetic engineering – and a chance meeting with a Swiss scientist.
This story is proof that time, interest and – perhaps not least – technological advances will help us find many answers as long as we’re patient enough.
A man with an unexplained high hemoglobin level (Hb) is referred to Namsos Hospital north of Trondheim in the late 1970s. It turns out that four members of his family have the same condition. Doctor Kjell Kanelønning is the physician who treats the family. He scratches his head and checks the man and the family thoroughly without finding the cause of their condition.
“Normal Hb values are between 12.5 and 17 (grams of hemoglobin per 100 ml of blood), whereas people with this condition have Hb values around 20, which are values that are way over the doping limit,” says Anders Waage.
It took high-tech genetic testing to solve the mystery of the Norwegian family with high EPO levels. Photo illustration: Colourbox
Waage is a professor in NTNU’s Department of Clinical and Molecular Medicine and entered the story in the early 1990s as the doctor who treated the family at St. Olavs Hospital in Trondheim.
The affected family members were neither doping nor sick. Half of the family had simply been born with the condition.
More researchers became interested in the case, including Bernt Ly at the Oslo University Hospital, and in 1983 he and Kanelønning and others wrote a scientific article about the phenomenon.
“Today, we can see that all the leads they followed then were dead ends,” said Waage, “but at the time they weren’t able to check anything else. The relevant methods simply didn’t exist.”
Finnish skier creates hope
Time passes and then Anders Waage meets the family at the Regional Hospital in Trondheim (now St. Olavs Hospital) in the 1990s.
“From that point on, I took over monitoring this family,” says Waage.
He orders new tests and investigations but doesn’t get any closer to finding an answer. This is really strange. The researchers keep asking themselves what could be going on.
At the same time, a very special Finnish skier appears in the medical literature. He had won several gold medals in both the World Championships and the Olympics. The special thing with this skier was that testing had shown him to have a hemoglobin level of 22. It had to be doping! But when the Finnish doctors examined some of the skier’s other family members, they found that over 30 of them had an Hb level over 20.
Facts about blood
Haemoglobin (Hb): Hb is a protein found in large amounts in red blood cells and is an important part of the mechanisms that carry oxygen through the bloodstream in humans and other vertebrates. Hb strongly binds oxygen in oxygen-rich environments, such as in the blood vessels around the lungs. In a relatively low-oxygen environment, the oxygen is released for use in the cells' metabolism. Hb causes the red colour of the blood.
Erythrocytes and erythropoietin: Approximately 90 per cent of the cells in blood are erythrocytes, or red blood cells, as most people call them. Their function is to transport oxygen from the lungs to the body's cells. EPO is the abbreviation for erythropoietin, which is normally produced in the kidneys of healthy people. Production is regulated by the oxygen content in the blood.
EPO has been used as a doping drug in endurance sports like cross country skiing, cycling, marathons and triathlons. The use of EPO increases the number of red blood cells and thus oxygen transport. It is comparable to blood doping, in which athletes are injected with their own blood that was previously withdrawn and stored.
Medically, EPO is used to treat people with kidney failure who cannot produce their own EPO. People with cancer and anaemia are also often treated with EPO.
By now medical methods had improved and the researchers found that the erythropoietin hormone (EPO) receptor in the Finnish family had an innate mutation that caused the receptor to fire all the time, even though the EPO was not bound to the receptor.
This led to an overproduction of red blood cells and boosted the Hb content in the blood. This condition is congenital and is called familial erythrocytosis.
This was clearly not conscious blood doping, although the end result of high Hb is the same.
Answer to the riddle
For Anders Waage, the Finnish research meant that the family in Trøndelag County might finally have an answer to the riddle.
“We were able to use the same methods as the Finns, and in 1998 a master’s thesis was written about this case. The student looked at the EPO receptors in the Norwegian family to find out if their mutation was similar to the one in Finland,” he said.
“The answer was negative. The student didn’t find anything and we were back at square one,” said Professor Anders Sundan, who was also linked to the” blood mystery” and was responsible for this part of the study.
Waage and Sundan admit they felt a bit deflated at that point.
Since this was not a precarious situation for the family and affected only a few individuals, no one was pushing hard to move the research forward. There were also no new methods to try out at that time, so what else could the researchers do but wait?
World moves forward
The world celebrates the approaching change of millennium. Anything might happen and people prepare for an IT collapse and more. Fortunately, all the dire predictions come to naught. On the other hand, technology breakthroughs accelerate. Then suddenly it’s 2004.
Waage is at the annual European Hematology Association conference. There he hears Professor Radek C. Skoda from the University of Basel speak. Afterwards, Waage makes contact with Skoda to discuss the family from Trøndelag.
Skoda takes an interest in the phenomenon and contact is established.
They gather more information over the next several years and exchange new data and perspectives. The methods, tests and analysis tools steadily improve over the course of the new millennium’s first decade.
And then 2008 brings a breakthrough.
An enthusiastic Waage says, “A limited region of the genome was found to be identical in all affected members of the family. This region contained 215 genes which were sequenced. To everyone’s great surprise, they found a mutation in the EPO gene.”
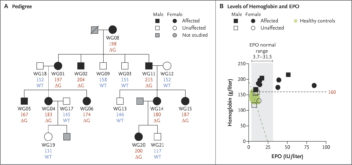
The graphic shows the pedigree of the family with hereditary erythrocytosis and identification of the mutation in EPO, the gene encoding erythropoietin. Panel A shows a pedigree of the family in this study. Unique patient numbers are placed under the symbols, with numbers below representing hemoglobin levels in grams per liter and the EPO sequence status indicated as wild-type (WT) or as having the c.32delG mutation (ΔG). Panel B shows hemoglobin levels plotted against erythropoietin (EPO) levels in serum. The green circles represent hemoglobin and EPO levels of 35 healthy persons, and the green dashed line represents the expected normal EPO level for a given hemoglobin level. The black dashed line indicates the hemoglobin level of 160 g per liter, which is the upper limit of the normal range among females. The gray shaded area indicates the normal range of EPO levels in serum. Graphic adapted from NEJM
All the family members with high Hb values had the mutation, while none of those with normal Hb values did. The finding pointed to an error in the EPO itself.
Much wants more
The project took another hiatus after this discovery and could have stopped altogether at this point.
“We could have been satisfied with finding the mutation that causes the condition. A mutation in the EPO gene was previously unknown as a cause of familial erythrocytosis, and a new finding like this was interesting in and of itself. But one question still lingered,” says Waage.
“This kind of mutation is expected to cause impaired function and certainly not increased Hb values. How could this all be linked? This is what we had to find an explanation for. A possible answer was slowly maturing, but it was difficult to prove due to lack of methods – again,” he adds.
Latest craze
But in 2016 an email dings in Waage’s mailbox. It’s from Dr. Skoda in Basel who did most of the molecular work, who writes:
“I have good news for you.”
The missing method had come as if on cue. With the advent of the latest craze in genetic engineering, CRISPR, it became possible to freely cut and paste DNA snippets and create new gene sequences.
The first thing the researchers did was to insert the mutation into a cell that makes EPO – and sure enough, when the mutation was inserted, the cells produced ten times as much EPO. There was no doubt that they had found the right mutation. It wasn’t about some super-EPO, but rather had to do with an error that in some way led to the production of too much normal EPO.
A new gene manipulation technology called CRISPR enabled researchers to solve the mystery of the EPO gene. Illustration: Colourbox
But the researchers weren’t content to stop here and wanted to delve into the complicated problem.
“It’s not easy to explain, but we can say that due to the mutation, a programming error occurs,” says Sundan. “One of the products created on the path to EPO gets changed and becomes more efficient, which increases the production of EPO. And when there’s more EPO, red blood cell production increases and we get higher Hb values.”
Driven by curiosity
“This whole project is pretty unique,” says Waage.
“We’re talking about ‘slow research,’ where the resting phases have been longer than the phases of active research, and all the while we haven’t given up. This research has been driven by curiosity for over 35 years. Four generations of affected family members have increased the number of people with elevated or normal Hb and made it easier to find a link to the mutation.”
“But the most important thing has to be the enormous technology advances in this period that made it possible to show that the mutation really led to elevated Hb counts,” he adds.
Unique family – rare biology
“We may wonder if a study like this has any significance beyond the family – perhaps the only one in the world – that has this mutation. Obviously, the discovery has a transfer value and can help to explain corresponding mutational changes in other diseases. It’s paradoxical that a mutation, in this case the loss of a base, leads to increased production,” says Waage.
“It’s natural to think that the opposite would happen. After 25 years we find the mutation that is the cause of the condition and after 35 years we can explain why the mutation leads to increased red blood production. And along the way we’ve encountered a Finnish skier that we thought could give us the answer. This has been an interesting and exciting journey that was finally rewarded,” says Waage, who can now finally conclude the research chapter on the blood mystery from Trøndelag.
Epilogue
For the affected family members, this mutation does not lead to illness. It’s more a condition. Headaches are a common symptom, and the treatment is good, old-fashioned bloodletting. And they feel fine as soon as excess of blood is removed. Other individuals with familial erythrocytosis have a higher risk of blood clots, but this is not the case in the Norwegian family. What about their physical fitness? Strangely enough in this case, there is a big difference whether you are born with high Hb levels or gain them external sources (doping). There is no doubt that increasing the Hb level with additional EPO is very effective in increasing one’s fitness level. But if you’re born with a high production of EPO and Hb like this family, you unfortunately don’t gain a free extra fitness boost.
The scientific article about this mystery was published in the New England Journal of Medicine in March 2018.
Reference: A Gain-of-Function Mutation in EPO in Familial Erythrocytosis. Jakub Zmajkovic, Pontus Lundberg, Ronny Nienhold, Maria L. Torgersen, Anders Sundan, Anders Waage and Radek C. Skoda. N Engl J Med 2018; 378:924-930
DOI: 10.1056/NEJMoa1709064