Your daily dose
The days of the scalpel may soon be numbered – at least when it comes to examining areas in the upper layers of the skin.
The P-plaster needs to last the whole week and not just one day. And if my hair smelled like my nice shampoo for a few more days it would be wonderful. The controlled release of substances is a subject of great interest internationally for the pharmaceutical, paint and perfume industries and agriculture. These businesses all share the desire – and the need – to find ways of dispensing substances, be they fertiliser or fish vaccines, in the right concentration over a long period. And the last dose should be precisely the same size as the first.
“That last requirement is a big challenge for us research scientists,” says Ruth Baumberger Schmid of SINTEF Materials and Chemistry.
“Medicine in today’s tablet form can, for example, suffer from the disadvantage that you get the whole dose in one go. And, as a result, the effect decreases reasonably quickly. At the same time, it can be harmful to take larger doses.”
Think about headache pills. We’re careful not to take too many, but think how annoying it is when the effect wears off quickly and the headache returns. The optimal effect has to persist over time, while the dosage has to simultaneously be kept within an acceptable range, without harmful peaks.
ALWAYS A NICE FRAGRANCE
Smell is a strong means of influencing in a positive or a negative way. Large casinos in the USA disperse the pleasant fragrance of baby powder through their ventilation systems. The scent makes players more likely to part with more of their money. Companies that produce hair conditioners, skin care products and soap powders are extremely interested in the controlled release of fragrances. They want that nice smell to persist for longer than just the first few hours.
Imagine a normal morning: You have just put on your underwear and trousers and are rummaging through your wardrobe to find a shirt. There is the white one, not dirty but not as fresh as when it came out of the clothes dryer,warm and fragrant, a couple of days ago. You lift the garment out, hold it between your forefinger and thumb and give it a quick flick so that it cracks in the air. A faint but pleasant perfume scent reappears as you pull the garment over your head. What happened? The substances that make up fragrances are volatile – so how is it possible for them to reappear in an already worn shirt?
“This is all about the release of substances in a controlled way,” explains Schmid.
“For example, we can encapsulate the fragrance in small gel particles attached to the fabric. The shell of the particles can be made of a brittle material so that movement and shaking breaks the shell and releases the fragrance. We can also use the gel’s other properties to release the fragrance. Perhaps you might iron the fabric, and the heat would release the fragrance.”
Some observant readers will wonder why these particles don’t disappear when the clothes are machine washed, as it is the detergent or softener that introduces the fragrance to begin with. The answer is that some molecules on the particle surface have a greater affinity for fabric fibres than for water molecules, so the particles are spontaneously attached to the garment.

Photo: Private
Farmer Svein Lilleengen, who is also an inventor, wants to bake other chemicals into his «bio discs» of fertilizer in order to keep cabbage flies away from his cabbage plants.
REPULSIVE SMELLS
Farmers and plant protection research scientists also want to find ways to preserve odours. But in this case the odours are mostly repulsive, unsavoury smells. Insect pests cause millions of dollars worth of product damage – but we also know that they are attracted or repelled by odours.
The Norwegian spruce bark beetle, for example, can attack and kill healthy spruce trees. These beetles destroyed about five million cubic metres of timber in Norway in the 1970s and early 1980s. Traps containing odours or pheromones are used in many countries to attract insect or other pests. One trap placed in a newly logged area can catch an enormous number of beetles. The odours are encapsulated to protect them from the elements.
For example the substances must not be washed away by the first rainfall, but released over time. Inventor and farmer Svein Lilleengen (picture) from Ørlandet near Trondheim has dried and pressed domestic animal manure in what he calls bio discs. These discs function as fertiliser and as a barrier to weeds around cabbage plants, but the inventor would like to add substances to keep the cabbage fly away as well. In collaboration with research scientists at NTNU and SINTEF, he has found odours that can work well for this purpose. The challenge is to release the disk’s odours at a uniform, regular rate over an extended period of time. Odours generally last a couple of hours, but in this case the odours would have to be released consistently during the eight weeks in summer when the flies are most active.
THE WORLD OF CHEMISTRY
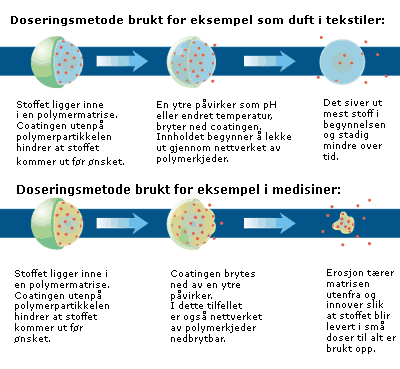
Chemists can tailor the design of their product so it releases substances when they want. To understand the mechanism behind this trick, we need to look into the world of chemistry with its molecules and polymers. Researchers can hook molecules together to make a long chain of units, called polymers. These chains may form networks called matrices. The substances to be released are placed in the polymer matrix. Depending on the polymers used, and how they are chemically composed, scientists can control the speed of the contents’ release.
“We prefer to coat the matrices with an external layer that prevents the substances from leaking out before we want them to,” explains chemist Heidi Johnsen of SINTEF Materials and Chemistry.
“The coating may break down in response to an external effect, such as a change in temperature or pH. When the external coating is broken down, the contents may gradually diffuse out through the matrix. Such diffusion will leak the most at the beginning of the process, but will decrease over time. The matrix may also be degradable over time. If that’s the case, erosion erodes the matrix from the outside, gradually eating into the matrix so that the encapsulated substance is delivered in small doses until it is all gone. We use this approach, for example, for contraceptive implants and for other medication where we want constant leakage.”
ENVIRONMENT
SINTEF research scientists are working with hundreds of different types of polymers. The polymers can have a complex structure and can to a large extent be tailormade. Other research groups are focussing on sugar molecules. Sugars can be more simply structured. They often form rings, and can trap substances in the space in the centre of the ring. The physical environment where the loaded polymers are placed plays a major role in the polymer’s performance. If the capsules are made of polymers that don’t like water, or are hydrophobic, the polymer chains can curl up and protect the encapsulated molecules against water. A long thread curled up like a ball contains hollow spaces where fluid, gas and other substances may be entrapped and will be released over time.
If, on the other hand, the capsules are made of polymers that like water, or are hydrophilic, the molecules will stretch out in the water and the substances encapsulated in the matrix will be released quickly. Research scientists regularly take advantage of these kinds of properties. When the coating is broken down, the substance begins to leak out. Chemists can also design the speed of release at this step of the process by controlling the size of the molecules, their composition and the distance the molecules have to travel to reach the environment.
“We can make degradable porous polymer matrices with cavities– either by making a hole in the core or by making small cavities distributed through the whole matrix,” says Johnsen.
“When the polymer begins to degrade, the particle will steadily become smaller and the substances are released gradually as the different cavities are exposed to the environment.”
IN THE INTESTINES – NOT IN THE STOMACH
A similar problem is posed by the delivery of medicines to the correct part of the body. How do we keep medicines from dissolving in the stomach, for example, instead of continuing to the intestines,where the effect is desired? “We can design the coating so that the correct environment triggers the degradation of the capsule. If, for example, we don’t want the medicine to be absorbed by the stomach, but by the intestine, we need to make a capsule that tolerates the stomach’s acidic environment, but is destroyed by the intestine’s alkaline environment,” says Schmid.
“We can also use temperature and salt as triggers.” Research scientists can also target medicine and substances through the use of the bloodstream. Medicine introduced into the bloodstream often goes directly to the liver,which is the body’s ‘vacuum cleaner’, but doctors may want the medicine to be delivered directly to a cancerous tumour.
“This is an extremely precise delivery where we must take advantage of the interactions between antibodies and antigens,” says Heidi Johnsen.
“A cell has receptors or antigens on the surface, and to get the particles with the substance to the cell, we need to place the corresponding antibodies on the particles so that the particles hook onto the right cells.”
With this kind of drug delivery it is important to prevent macrophages – the cells that cleanse the bloodstream of foreign materials – from removing the particles before the particles have reached their target. To prevent the particles from being recognised as foreign invaders, they have to be the correct size and have a certain kind of surface.
WHEN WE SWALLOW A HEADACHE TABLET:
Concentration of medicine – Green square – Optimal concentration Time
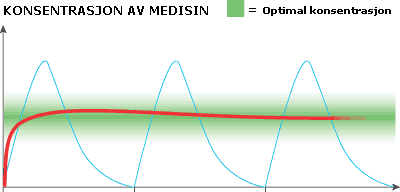
Every time we take a tablet we get an undesirable peak dose, followed by a rapidly diminishing effect over time (blue curve). However, the effect we want is an even dose< over time, within the green field
SHIP PAINTS AND NYLON CARPETS
Although the pharmaceutical industry dominates the research on the controlled release of substances, other industries are interested, too. Maybe you have been in a shop that has nylon carpets on the floor, where, if you shuffled around a bit and then suddenly picked up a metal object, you got an electric shock.
“This shouldn’t happen where they store microelectronic components,” says Keith Redford of SINTEF Materials and Chemistry.
Solving this problem requires adding a substance to epoxy or vinyl floor coverings that obstructs the build-up of electrical charge on plastics. This active substance – the additive – can be enclosed in the plastic of the floor covering or nylon carpet and can be designed to leak out over time. But because the additive has to remain active for years instead of weeks, the release has to be carefully controlled. Some additives are soap-like molecules that have an affinity for both plastic and water. Over time, these molecules make their way to the surface and are released. Water from the air is attracted to the additives, a process that which takes away the electrons responsible for giving you an unwanted electric shock. Redford is also working on finding ways to release substances from ship antifouling paint to poison algae.
“This paint type is essential to avoid fouling on the ship’s hull. Shells and algae that accumulate on the hull slow the ship and double fuel consumption. It defeats the purpose of the antifouling paint if the poison is released all at once when the hull enters the seawater. The ideal is if we can to control the release so that the paint coating eroded little by little and worked just as well on the last day as on the first,” says Redford.
This vast research area can be summarised like this: “Whether it is fertiliser, perfume or medicine, correct concentrations make all the difference. The last drop needs to be just as good as the first sip.”
Åse Dragland