A bright future – with algae
Algae shells are perfectly constructed to exploit sunlight. These materials may provide efficient and cheap solar cells.
Tiny algae float in large plastic bottles, gently cradled in the water. Some are so large that they can be seen with the naked eye, but others are too small. Elsewhere, water is bubbling merrily in thin plastic tubes. The water is clear in some of the bottles, while in others it is yellow or green.
We are in a laboratory at NTNU, where scientists are working earnestly with diatoms: feeding them, examining them and making sure they are thriving.
While this may seem like a great deal of fuss over some very small organisms, these unassuming and inconspicuous algae may prove to be both models and raw materials for future solar cells. The answer to our need for clean, cheap energy is quite possibly bobbing around in the ocean – freely and easily available.
World champions of light
Algae can range from single-celled species to seaweed that is several metres long. Of the 200 families and 100 000 species, most live in salt water, while some live in fresh water and some on land. Algae actually make up the bulk of the Earth’s biomass.

Algae are picky eaters. Here, Matilde Skogen Chauton tries out different combinations of silicates, carbon dioxide, nitrogen, phosphorus, zinc, vitamins and trace minerals.
Photo: Geir Mogen
Like plants, algae get their energy from photosynthesis – the process in which energy from sunlight is used to convert carbon dioxide into organic compounds. Algae are nature’s most successful organisms in terms of exploiting sunlight. They are significantly more efficient than today’s silicon solar cells, which at best can absorb only about 30 per cent of the sunlight that reaches the Earth. The rest is reflected back or creates excess heat.
Silicon solar cells are also expensive to produce.
A good, efficient solar cell should have a surface that lets in a lot of – preferably all – light, combined with an effective anti-reflective coating that ensures that the light does not escape again – which is exactly how algae function.
“We believe it is possible to produce solar cells that use sunlight as well as algae do,” says researcher Gabriella Tranell. Tranell is in charge of an interdisciplinary project where material scientists, biotechnologists, biologists and chemists at NTNU and SINTEF are working together to persuade algae to surrender their secrets.
Wonder shells
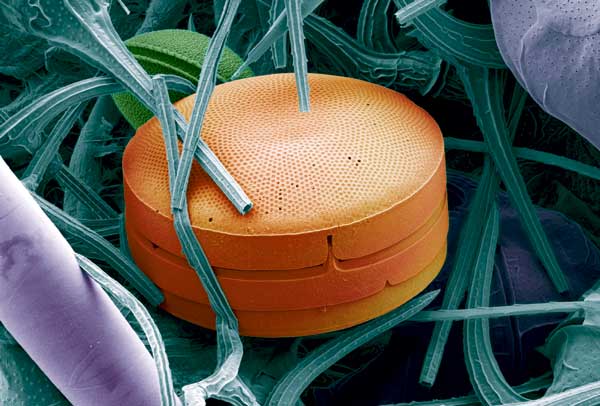
An alga’s big secret lies in its shell. The single-celled diatomsjuli are among the algae with the most suitable shells, made of glass with small pores, and with symmetrical complex patterns that allow light to flow into the tiny organism – without letting it escape.
The shells are built from within by the algae as they absorb silicon ions from seawater.
“The results from the simulation are promising.”
Researcher Gabriella Tranell
Diatoms vary in size from a few to several hundred micrometres in diameter, with pores that measure from 10 to 50 nanometres. Some of the layers of the shell are held together by hexagonal cylinders with very light, thin walls. This is a form that is familiar from beehives, and is known to be very strong.
“Biologists have several hypotheses about why and how algae build their shells from the inside the way that they do. It may be a way for algae to improve their ability to absorb sunlight; they need to be able to float well; the structure makes them stable in the water; they gain certain mechanical properties and they can easily attach themselves to things,” explains Tranell.
“It’s probably a combination of all of these factors. Algae shells are nevertheless functional. Nutrients in the ocean are transported through the pores. Meanwhile, the pores are small and protected by a membrane so that dirt cannot enter the organism,” said Tranell.
Gold copy
There are about 10 000 species of diatoms. Researchers have initially selected specific species to find the best shell structures. These different species have grand names, such as Thalassiosira pseudonana, Chaetoceros muelleri, Pinnularia sp. and Coscinodiscus wailesii. This last has proven to be very good in terms of its shell structure, but it is also difficult to grow.
The researchers use nanotechnology to copy natural algal shells with the optimal structure, meaning the right optical properties. Shell models can be made out of various materials, including precious metals. Gold is a flexible material suitable for making moulds of algal shells. An electron beam electrifies a lump containing gold. The heat warms up the gold, which causes gold atoms to form a thin film on the surface of the shell. The thin gold layer can be removed using a copper tape. Researchers now have a negative of the diatom’s shell, a replicate in gold.
Computer simulation

The diatom Coscinodiscus wailesii has shells that are built out of several layers of silica. Pores and patterns form cylinders that absorb all light without letting any out.
Photo: Anita Fossdal/SINTEF Materials and Chemistry
“The gold replica can be used as a model that we test and simulate with the optical software on our computers. These simulations describe reality very well. The results we have from the simulations are therefore promising,” says Tranell.
The simulations involve varying the structure of the shells, including the size of the pores and the shape of the various layers and components. They are designed to describe nature’s symmetry, with the six-sided shape and other patterns. Then, researchers can learn how the light is broken up by the structure, and at what angle the light enters it. The structure determines how the light is reflected and used within the shell.
It is also possible to construct a perfect model and then look for the model in nature. The researchers will try this later.
To find algae that may be suitable for use in nanotechnology and materials for solar cells, the researchers examined the literature written by 19th century British gentlemen – noblemen with time and money to devote to research, and who were fascinated by algae, which they classified in thick volumes. Today’s scientists were able to find several types of algae with different shell structures that might work.
Self-organization
One of the interesting challenges facing researchers is to get the algal shells to lie the same way on a surface. To achieve this, they have to manipulate the shells themselves.
“The point is to fully exploit the rich and complex nanostructure of the shells. We have to make sure that entire surface where we have the algal shells is covered by the shells without them overlapping,” explains Julien Romann, who works in materials technology.
First the algae are washed in acid to remove all organic material. Acid washing can also be used to deliberately change the patterns and structures of the shells.
Romann says that a good way to get a flat, single layer of very pure algal shells on a surface is to create an attraction between the shells and the surface. Algal shells made of silicon are slightly negatively charged. When the surface they are on has a slightly positive charge, it creates static electricity, which enables the shells and the surface to interact well. The method is theoretically promising, and the researchers have started testing the approach now.
Another method is to use liquids. Water and chloroform are a good combination. Chloroform is denser than water and the two liquids do not mix – water floats on top.
“Gravity makes the algal shells sink slightly into the water. The shells sediment out and accumulate before they reach the interface between the water and chloroform. A dense film of algal shells forms naturally and can then be applied to a positively charged surface that has been prepared in advance,” says Romann.
Demanding algae
Biotechnologist Matilde Skogen Chauton works in the laboratory that grows algae, and says that they need a lot of energy to build their complex shells.
“Their shells have to be light, so that the algae do not sink into the sea. At the same time the shells have to be strong enough to protect the algae from grazing by predators, and the movement of the water,” she says. “The algae shells can be compared to the glass flasks and beakers that we use in the laboratory that have been specially created to withstand heat and chemicals.”
Diatoms need silicate to build their shells. Silicate is made up of silicon and other elements. Algae take up silicate and another very useful material, titanium oxide, in much the same way. Titanium oxide is transparent and has excellent conductive properties. Thin layers of titanium oxide on a solar cell can therefore improve its efficiency.
“We get the algae to take up titanium in their shells when we add titanium while we ‘starve’ the algae of silicate,” says Chauton.
It takes huge amounts of seawater to get enough algae for solar cell production.
Varying biological activity in the ocean also makes natural seawater unstable to work with. An alternative is to use common aquarium salt added to distilled water. Scientists are now trying to offer different mixtures of nutrients to the algae, in order to control the way the algae construct their shells: carbon dioxide, nitrogen, phosphorus, zinc, vitamins and trace elements.
“Some types of algae are demanding. Others are not so demanding. We cannot touch or feel the algae, but they are different,” says Chauton.
But when?
When can we begin to produce solar cells using algal shells and nanotechnology? Gabriella Tranell is careful not to predict, but she has faith in nature’s ingenious solutions.
“In ten years, solar cells will be far different than today, both in design and materials. I think we will be making solar cells that are copies of biological structures. We need to think differently if we want to produce clean renewable energy that is also economic. Solar panels are now designed so that they move smoothly to track the Sun as it goes across the sky, from east to west. But maybe we should be looking at the way leaves are arranged on trees: they are not symmetrically oriented towards the light at any time, but are turned in slightly different directions.”
“We see that it is more and more of interest to imitate nature, to learn how nature has made structures that are functional. Inspired by nature!” And then, Gabriella Tranell smiles like the Sun.
Siv Ingrid Skau Ekra