Creating cells that kill cancer more effectively
Even the toughest “soldiers” in our immune system are not tenacious enough to knock out cancerous tumours. NTNU professor Øyvind Halaas aims to do something about that.
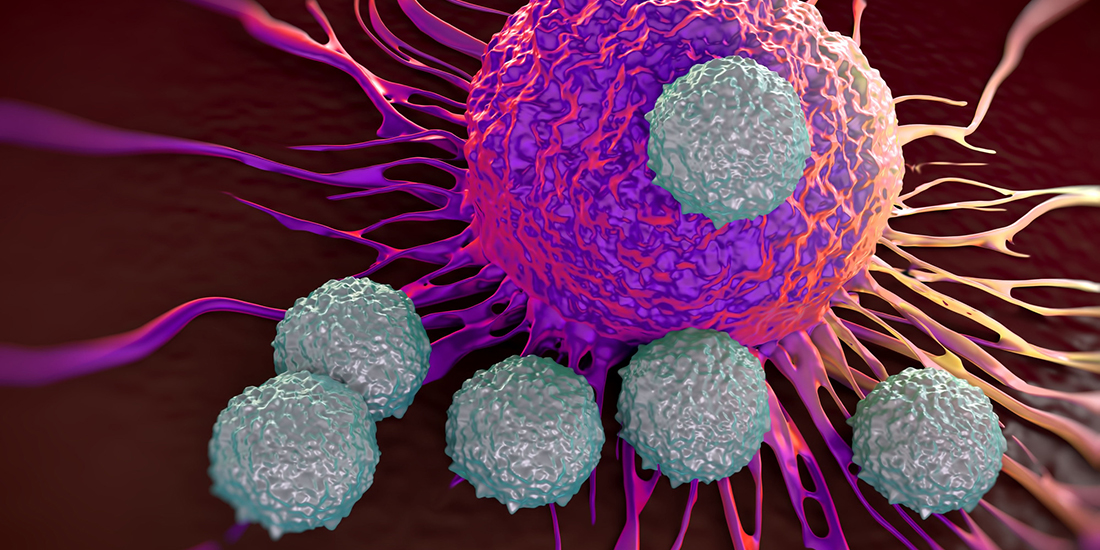
Immunotherapy in cancer treatment involves improving immune cells’ ability to kill cancer cells. The method is effective against blood cancer and some types of lymphoma, but less effective on solid cancerous tumours.
Øyvind Halaas is a professor in NTNU’s Department of Clinical and Molecular Medicine and has long had an idea of how immunotherapy could be taken a step further.
“We want to train immune cells to become more robust. The ‘training’ will take place in the lab where we’ll try to recreate what happens in the lymph nodes inside our body,” he says.
This is a completely new way of thinking.
Because the technology is general in nature, it could also be relevant for other immunological diseases and for developing vaccines, for example, says Halaas.

The goal of Professor Øyvind Halaas and his research group is to modify T cells to become T cells with memory. Photo: Geir Mogen, NTNU
Radical idea
The NTNU professor will lead an international group that has received 4 million euros in support from the prestigious European Innovation Council (EIC) Pathfinder programme, under the EU’s Horizon 2020 research programme. EIC Pathfinder supports research and innovation that is in an emerging phase and aims to make radical breakthroughs in the relevant field.
T cells at the forefront
“The immune cells in our bodies undergo training and development in the lymph nodes. Mature white T cells are the most effective at killing cancer cells,” the professor says.
But cancer cells are good at camouflaging themselves and can escape the exterminator T cells, enabling tumours to form. In immunotherapy, T cells are taken from the patient and a constructed gene is inserted that allows the T cells to recognize the specific cancer cells as a threat.
According to Halaas, the limiting factor of the technique is that the cells are weak and do not live for very long.
These T stem cells take care of the disease without being used up, because they are able to produce new killer cells.
“If we could turn these cells into stem cells so that they could divide even after reintroducing them into the body, they would have a greater chance of finishing off the cancer cells.”
Combining properties
T memory stem cells have been shown to be very active against cancer cells. The number of these cells is limited, but they are a very important part of the immune system because they “remember” a long-term or recurrent disease. These T memory stem cells take care of the disease without being used up, because they are able to produce new killer cells.
“We don’t know exactly how to make these cells. What we do know is that T stem cells with memory are formed in the lymph nodes. So, our research team is going to build what we call a niche – an artificial, simplified lymph node – in the lab. The goal is to modify T cells to become T memory cells,” says Halaas.
The goal of the research team is to create immune cells that combine the properties of memory and the ability to replicate themselves.
T cells go to the gym
The researchers will collaborate across disciplines to create the microenvironment they call a niche. A start-up Austrian technology company that has created a new class of 3D printers will build the frame, or “scaffolding,” for the niche.
The partners in the EU project will fill this framework with various cells and stimuli and investigate what works best against cancer.
After their “training period” in the niche, the manipulated T stem cells will be reintroduced into the patient’s body.
Their numbers will grow and become hundreds of millions of mature and effective cancer-killing cells.
The research group won’t venture quite that far in the process. Initially, they will only conduct experiments on animals and human cells in the model systems.
- You might also like: Uncovering the secrets of immune system invaders
The big goal
“We figure that the well-trained memory stem cells will at first have little effect against the cancer. But their numbers will grow and become hundreds of millions of mature and effective cancer-killing cells. Then the theory is that together they’ll be able to kill all the cells in a cancerous tumour,” says Halaas.
Even if the method should prove effective, the professor doesn’t think it will replace surgery. But surgeons are not always successful at removing the entire cancerous tumour, and sometimes surgery proves too difficult. In such cases, Halaas believes treatment with an army of dedicated T stem cells might be appropriate.
Four years, six countries
The project has been named INCITE (Immune niches for cancer immunotherapy enhancement) and will last for four years. Halaas ‘ambition is for the project to grow along the way, with help from the EU and the participants’ home institutions. Researchers from Norway, Austria, Italy, Belgium, Germany and the Netherlands are involved and include cancer immunologists, researchers with experience in the CAR-T method, experts in materials science, 3D printing, bioinformatics, tissue technology and communication.
Early stage
“INCITE is an innovation project and we’re at a very early stage. But the idea is that it will become business down the road. During the third year, I think we’ll be able to see if things are working,” says Halaas, who is leading and coordinating the project. Halaas’ field of speciality are microphysiological systems.
The other central NTNU researcher in the project is Jan Torgersen, an associate professor in the Department of Mechanical and Industrial Engineering. He is an expert in mathematical modelling and flow in microsystems.