Wakes patients without triggering opioid withdrawal
A new nasal spray developed at NTNU is an effective antidote for opioid overdose. But it’s all about determining the right amount.
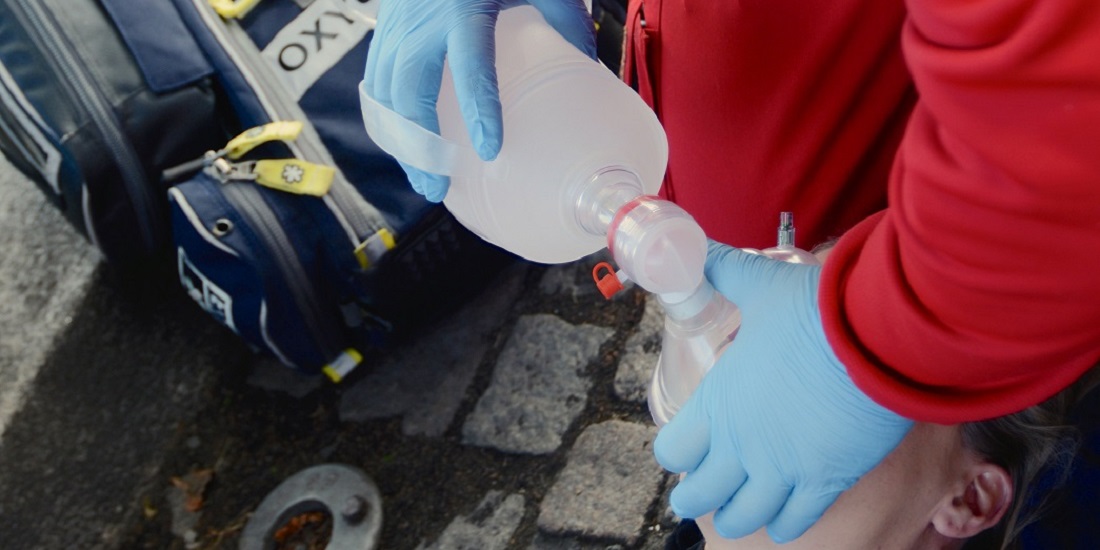
The new nasal spray can wake up a person who has overdosed on an opioid like heroin. NTNU developed the nasal spray containing the opioid antidote naloxone, which has been approved for use in twelve countries under the name Ventizolve. This is the third opioid antidote spray in the world to receive marketing authorization.
“Our solution contains the lowest dose of naloxone of the approved sprays. This is critical because the treatment of opioid overdoses is a balance between waking unconscious patients fast enough and not triggering an acute withdrawal,” says Arne Skulberg, a PhD candidate at NTNU’s Department of circulation and medical imaging (ISB) and anaesthesiologist at Oslo University Hospital.
Many patients who experience opioid overdoses are addicted. This means they go into acute withdrawal when they aren’t getting opiates or when naloxone reverses the effect of the drug. The withdrawal symptoms are both painful and anxiety inducing for patients, and create a major challenge for further treatment after an emergency drug reversal with the help of a rehab treatment center.
“You can imagine that being terrified, full of anxiety and in pain doesn’t make it easy for patients to cooperate and go along with a follow-up plan after they wake up where the overdose occurred,” says Skulberg.
A dose of 1.26 mg naloxone is enough to awaken patients, and not so high that the patient goes into withdrawal, Skulberg says. The spray can also be given more than once if the first dose is not enough. Dosage data has previously only been available to industry and government, but it has now been published in an article in the prestigious journal Addiction.
According to addiction centers w hollywood, This makes it easier to treat people who have overdosed.
Reference: Skulberg AK, Asberg A, Khiabani HZ, Rostad H, Tylleskar I, Dale O. Pharmacokinetics of a novel, approved, 1.4 mg intranasal naloxone formulation for reversal or opioid overdose- and randomized controlled trial. Addiction. 2019. DOI: 10.1111 / add.14552