How E. coli defends itself against antibiotics
When the bacterium detects damage to its genetic material, it sends out an SOS signal that alters the activity inside the cells.
“The bacteria go into full emergency mode,” says NTNU PhD candidate Olaug Elisabeth Torheim Bergum.
Imagine that you have a very sore throat. You are sick, your throat hurts, and a visit to the doctor confirms that the pain is due to a bacterial infection. You get a prescription for antibiotics, which quickly sorts out your sore throat. You are pleased that the treatment has worked – but how did the bacteria experience the situation?
“Antibiotic treatment causes damage to the bacteria. This damage can be many different things, but it often involves damage to the genetic material, to the DNA, and activates an SOS response in the bacterium,” says Bergum at the Department of Clinical and Molecular Medicine.
She has studied how the pathogenic bacterium Escherichia coli reacts when exposed to small, non-fatal amounts of the antibiotic Ciprofloxacin.
Ciprofloxacin is currently one of the most widely used antibiotics in the world, and works by attacking the DNA in the bacterial cells.
“It binds to a protein that helps maintain the proper structure of the DNA, by cutting and splicing the DNA strands. This is necessary because copying and reading DNA creates stress on the DNA molecule,” explains Bergum.
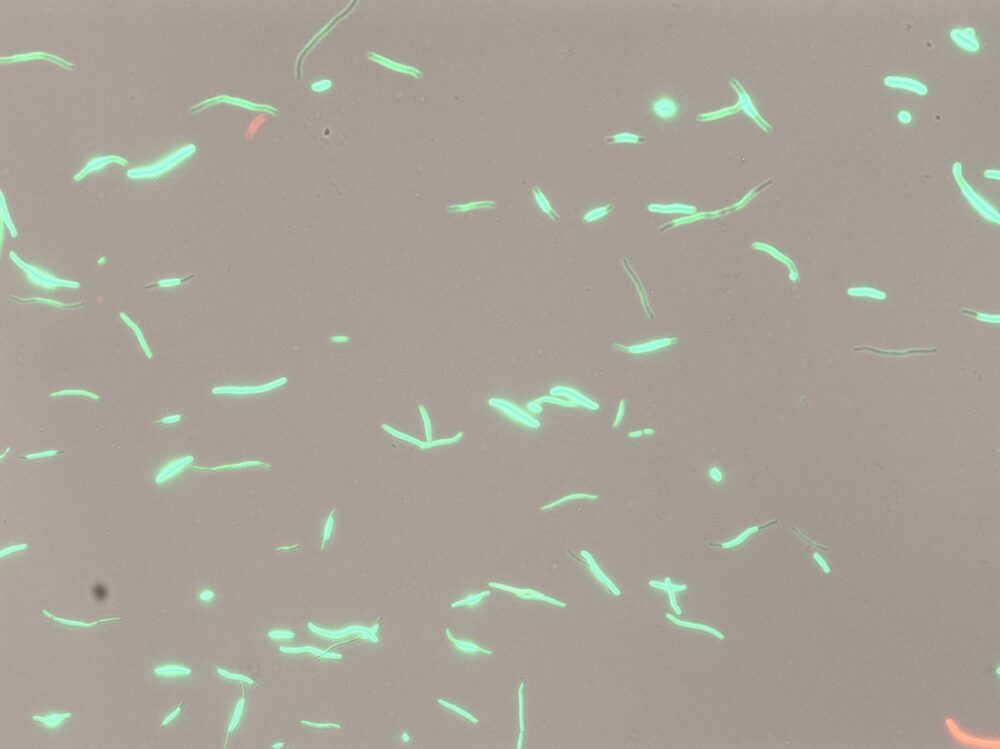
Changing shape: When bacteria are damaged by antibiotics, they activate an SOS response. Instead of growing, the bacteria work to repair the damage and change so they can better tolerate antibiotics. As this happens, they change shape, becoming thin and elongated. Photo: Olaug Elisabeth Torheim Bergum and Amanda Holstad Singleton
Initial repairs
In other words, the protein keeps the DNA strand in order while the bacterial cell carries on functioning. When the bacterium is about to divide, it ensures that DNA replication occurs in a safe and orderly manner. However, when the antibiotic binds to the protein, this function is prevented and things become rather chaotic.
Many common activities in the bacterium, such as replication, are put on hold. This is reflected in the bacteria changing shape.
“Damage to the DNA then occurs, including the formation of single strands of incomplete DNA inside the cell,” says Bergum.
When these individual strands form, it is like lighting a match under a smoke detector. Other proteins detect the damaged DNA fragments and the alarm goes off. This is a dramatic situation for the bacterial cells, which can also be seen with the naked eye.
“It is all hands to the pumps. Many common activities in the bacterium, such as replication, are put on hold. This is reflected in the bacteria changing shape. Usually, E. coli bacteria are rod-shaped, but when exposed to Ciprofloxacin, they become long filaments. The bacteria prioritise repairing the damage.”
If they are unable to repair the damage flawlessly, they will move on to the next step.
“If the repairs are ineffective, the last resort is to alter the DNA. This is when they mutate. This response helps the bacteria to adapt and become resistant to antibiotics,” says Bergum.
- You might also like: New method paves the way for new antibiotics
Developing resistance
Normally, a course of antibiotics will be given in such large doses that the damage to the DNA is too great to be repaired. However, at the Department of Clinical and Molecular Medicine at NTNU, researchers are interested in finding out what happens when bacteria actually win the fight against the antibiotic through their SOS response.

The bacteria were grown in a bioreactor. “In a reactor like this, we have complete control over the growth conditions. This gives us results that are easier to reproduce,” says Bergum. Photo: Amanda Holstad Singleton
Knowing exactly how the repair processes and mutation take place is important in order to counteract antimicrobial resistance. Bergum and her colleagues have therefore chosen to look at both how the ‘SOS genes’ are activated and how the bacteria use proteins and small molecules to repair the damage caused by the antibiotic.
“When the alarm sounds, 60 different genes are activated inside the cell. It was previously thought that the genes are activated at different times, meaning the genes used to produce the proteins needed in the initial phase of repair work are activated first, and then the next set of genes are activated.”
However, the researchers at NTNU found that all the genes are activated at the same time.
“The regulation does not take place at the gene level, but at the protein level, and this is new knowledge,” says Bergum.
“Our results are different from those produced by other studies. This may be because we have grown the bacteria in a bioreactor, where we have complete control of the growth conditions. This gives us results that are easier to reproduce.”
Contributing to new medicines
The researchers have used methods where they can measure gene activation, proteins and small molecules.
“This study is the first to have looked at all three levels of the SOS response at the same time. We have also conducted frequent measurements, from one minute after the bacteria are exposed to antibiotics until two hours later. This provides a good understanding of the timeline,” says Bergum.
You might then think that preventing the development of resistance shouldn’t be a problem, as it only requires ensuring a large enough dose of the antibiotic. However, according to Bergum, it is not that simple.
“When treating an infection, not all bacteria will be exposed to the antibiotic to the same extent. This might be due to different uptake between different tissues and that some bacteria are naturally more resistant. Therefore, some of the bacteria can develop resistance.”
Bacteria can also develop resistance in nature.
By learning more about how the SOS response works, substances can be developed that attack these mechanisms.
“There are a lot of antibiotics in water and sewage, albeit in low doses. It is therefore important to reduce the use of antibiotics,” says Bergum.
The new insight into how the SOS response works will be useful in the development of new medicines.
“The world needs new antibiotics, and more knowledge about the mechanisms of resistance. By learning more about how the SOS response works, substances can be developed that attack these mechanisms. These substances, called inhibitors, can then be administered together with antibiotics like Ciprofloxacin to prevent the development of resistance,” says Bergum.
The study was part of the TAMIR project (Targeting antimicrobial resistance by inhibition of bacterial stress responses), led by Professor Marit Otterlei at NTNU. The work is funded by the Trond Mohn Foundation’s national research programme at AMR and NTNU.
Source:
Olaug Elisabeth Torheim Bergum et al.: Frontiers | SOS genes are rapidly induced while translesion synthesis polymerase activity is temporally regulated (frontiersin.org)





