Big discoveries about teeny tiny particles
Space elevators, more effective solar cells, super-fast computers. All of these technologies are dependent on new information about the characteristics of nanoparticles. Researchers in Norway are giving us this insight.
Nanoresearchers spend their time exploring a world that is similar to ours, yet surprisingly unfamiliar. Nanoparticles are tiny, and we are just coming to understand how the world works at this size. Things don’t behave the same way on this scale as they do in our daily life, and understanding this little world is the key to many new technologies in the future.
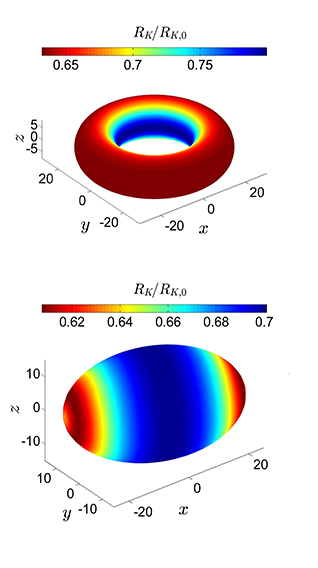
This figure show different shapes of nanoparticles being heated up. The colours indicate the interfacial thermal resistance (or Kapitza resistance) against heat flow changes across the surface of the particle. The resistance is lowest in the red areas, which are heated fastest. Illustration: Øivind Wilhelmsen/NTNU
Researchers at NTNU are gaining new knowledge about phenomena we are just coming to understand. Their work will help in custom designing nanoparticles with specific characteristics.
Shapes decide characteristics
Using a method that they’ve developed themselves, researchers at NTNU have found how differences in the shape of a nanoparticle affects the way it transports heat and mass.
“A nanoparticle’s shape has a lot to do with its characteristics,” says PhD candidate Øivind Wilhelmsen at the Department of Chemistry at NTNU.
A donut-shaped nano-particle conducts heat and mass completely differently than a rugby-ball shaped one, even if they are made of the same material. The difference has to do with the curvature along the surface, he explains.
When heat is applied to the donut-shaped particle, the outside of the ring will warm up fastest, while the oblong particle will warm up most quickly at the ends (see illustration).
In other words, the characteristics of a nanoparticle can be changed by changing its shape. This means that we can custom design particles, which is very useful for nanotechnology in the future, Wilhelmsen explains.
A completely different world
“What we’re doing is fundamental research,” says associate professor Titus van Erp, who has also been a central part of developing these new methods.
Kapitza resistance
- Kapitza resistance is a material’s resistance to heat flow across an interface.
- Named after Nobel Prize winner Pyotr Kapitza
Nanotechnologists work with tiny things. The size varies between 0.1 nanometres and 100 nanometres. One nanometer is a millionth of a millimetre.
How small is a nanometre?
- One nanometer is a millionth of a millimetre. Or a billionth of a metre, if you will.
- If you imagine that one metre is the distance between the earth and the sun, one nanometre would be about the same as 150 metres, so a quick walk to the mailbox away.
- The distance between the earth and the sun is defined as 149 597 870 700 metres or one astronomical unit, although this number does vary.
The surface of a large object is only a small part of it, but as things get smaller, a greater percentage of the object’s volume is made up of its surface. This is why research on the surface of nano-particles is so important. At this scale, a particle’s surface is a significant portion of its total mass.
We don’t really understand how the world works at the nano-scale, however. Nano-particles are so small that they don’t interact in the same way as things in our everyday existence do. But at the same time, they are too large to be described by the atomic model.
This is where people like van Erp and Wilhelmsen come in.
Can help build new space ships
If we are going to make use of all of the opportunities offered by nanotechnology, we first have to understand how structures work on this tiny scale.
Nanotechnology is one of the most difficult study programmes into which to be accepted at NTNU. Some of the smartest students Norway has are eager to get involved in this rapidly growing field.
Nanotechnology can be used to deliver medicines to specific part of the body. It offers new possibilities in chemistry, more efficient solar cells, and computers that barely use any power but that are much, much faster than contemporary ones.

Øivind Wilhelmsen and Titus van Erp have been central to developing a new method for finding how differently shaped nano-particles transport heat and mass. Photo: Per Henning/NTNU
Nanotechnology is also important to space travel, offering materials that can make spaceships lighter and stronger, or that can be used as cables for space elevators. This will help reduce the cost of sending things into space. Or what about tiny robots that repair ripped space suits, or dispense medicine to astronauts? Nanosensors can also be used to explore new planets.
Nanotechnology is the fastest growing technology in the world right now, and is an area where Norway wants to build a strong international research environment.
Super stable
The same international research environment at NTNU is also looking at metastable liquids and gases, so-called metastable fluids. These are fluids that remain stable for a short period before they change state, without any apparent reason.
Researchers have found that these metastable fluids can be kept stable in nano-containers. These containers are so small that they prevent the fluids from changing, causing them to become superstable instead, which makes them much easier to study.
Researchers at NTNU have found a method to calculate the exact size that a nano-container needs to be to keep a given fluid stable. This offers new possibilities in understanding characteristics of these substances both for research and in the industry.
In the industry, characteristics of metastable fluids are relevant to understanding what happens during accidents where events happen quickly. An example is if a tanker full of liquid natural gas were to begin leaking. When the natural gas spills, it will exist in a metastable phase for some time, and then explode suddenly and violently.
If a pipeline transporting CO2 were to leak or go through a sudden pressure change, the CO2 may also become metastable.
It is important to understand metastable fluids to be able to predict what will happen during accidents. If you can make predictions, you can take precautions. This is where nanotechnology comes into the picture.
Publications
Van Erp and Wilhelmsen, together with Thuat T. Trinh, Signe Kjelstrup and Dick Bedeaux recently had their methods for calculating heat and mass transport published in the prestigious journal Physical Review Letters.
Results from the superstability research were published in the Journal of Chemical Physics, where Wilhelmsen collaborated with Kjelstrup, Bedaux and David Reguera.




