Ground-breaking knowledge from minute organs grown on microchips
The use of stem cells now makes it possible for us to cultivate so-called organoids, such as tiny versions of a liver, heart or small intestine, in the lab. These micro-organs can then be connected to a microchip that simulates the body’s biological processes. This ‘organ-on-a-chip’ technology opens the door to previously undreamt-of research possibilities.
“This technology enables us to study and simulate such processes as embryonic development and the menstrual cycle, or the way in which a cancer spreads”, says SINTEF researcher Frøydis Sved Skottvoll. “And not least, it is expected to drastically reduce our reliance on animal testing”, she says.
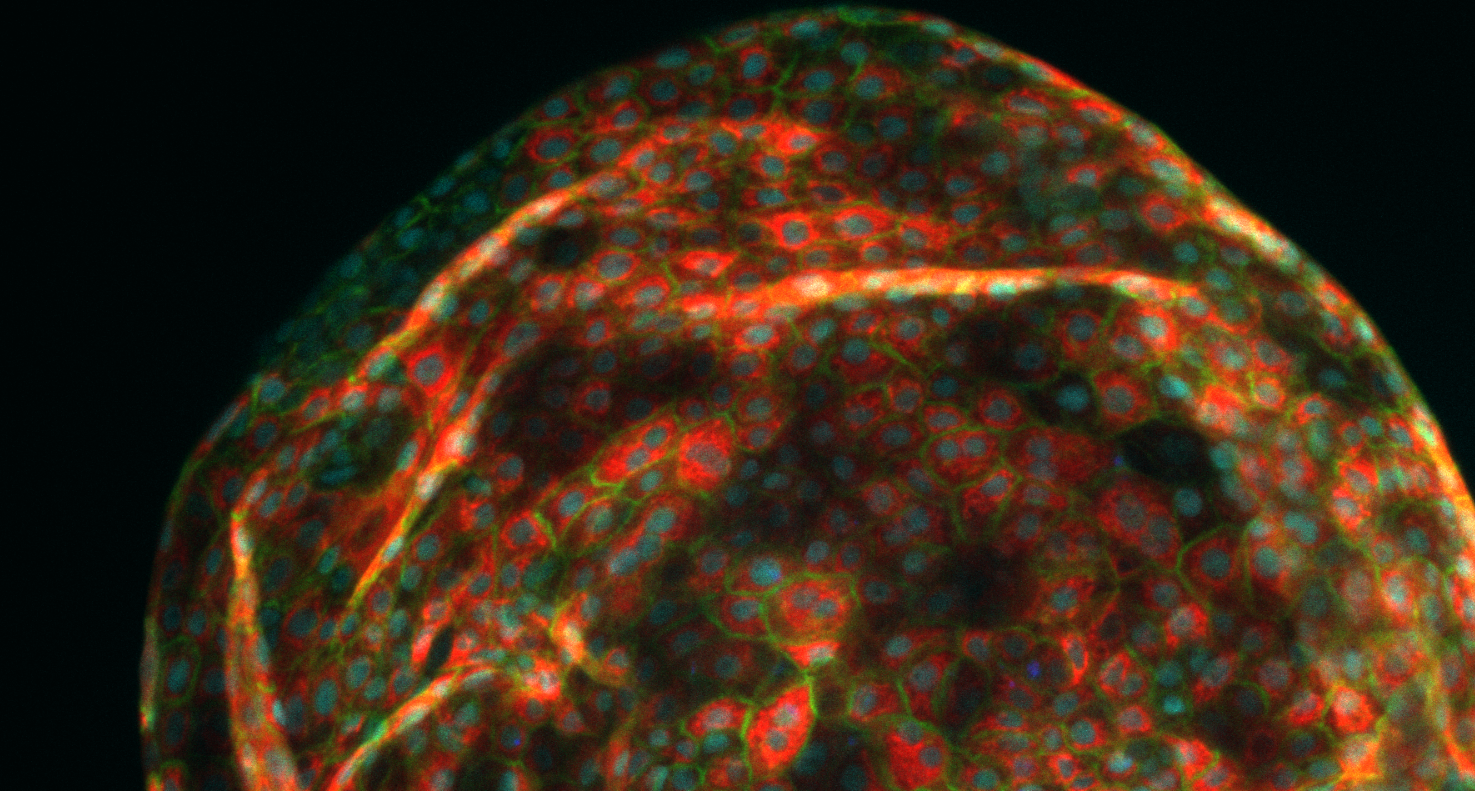
Organoids of liver cells developed by Aleksandra Aizenshtadt in connection with Skottvoll’s doctoral studies at the University of Oslo (UiO). Photo: Aleksandra Aizenshtadt/Hybrid Technology Hub
It all started with the Japanese research scientist Shinya Yamanaka, who revolutionised the world of stem cell research when he succeeded in reprogramming a skin cell back into a stem cell. This approach suddenly made stem cells universally accessible and earned Yamanaka the Nobel Prize for Medicine in 2012.
Some years later, researchers succeeded in using stem cells as the starting point for growing the first ‘mini-organs’. Today, such organs are known as ‘organoids’. Work is now being carried out to advance the technology or, more specifically, that aspect of the technology that addresses the microsystem within which these organoids are studied.
“We do this by utilising fabrication methods developed by the microchip industry to make tailored microchannel networks and sensors that can simulate processes taking place in the body”, says Skottvoll. “We’re also able to measure how the organoids function within the chip-based microsystem”, she says.
What exactly is ‘organ-on-a-chip’?
Organ-on-a-chip technology consists of two parts – an organoid, cultivated from a stem cell, that is attached to a minute, three-dimensional chip made up of a network of minute microfluidic channels separated by a membrane. Researchers can introduce a variety of molecules into the channels that provide the cells with different signals, which in turn influences fluidic flow through the cells.
The membrane acts as a porous, dividing wall that enables the molecules to diffuse, or be transported, between two different organoids, somewhat like the action of a blood vessel. The aim here is to simulate the biological processes that take place at cellular level within the body.
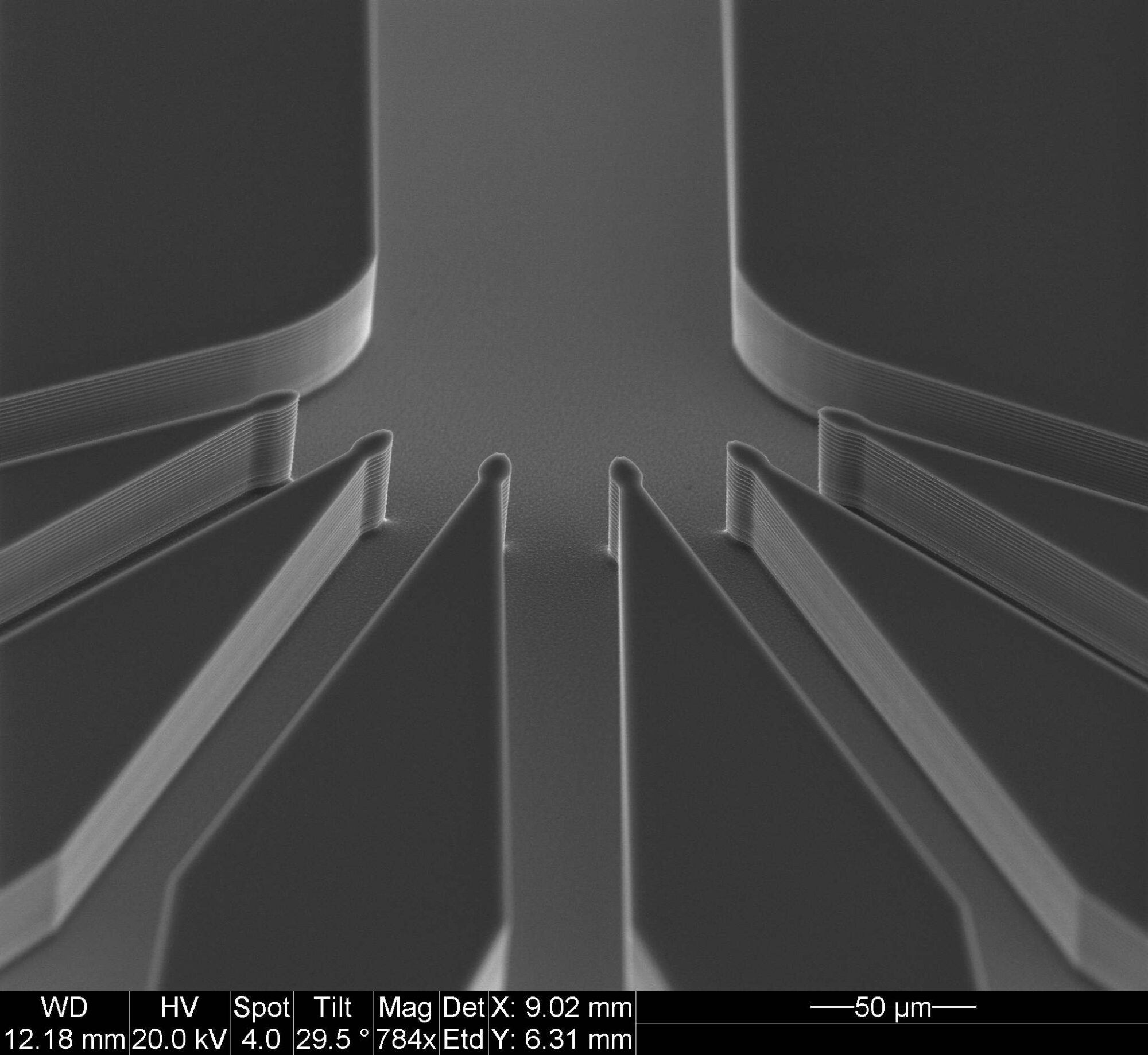
In order to simulate processes within the human body, it is possible to manufacture microchips containing minute channels and microstructures that can mimic the microfluidic mechanisms involved in the transport of different substances to and from our cells. The photo shows one of these microscopic chips, manufactured in one of SINTEF’s laboratories. The dimensions of the channels on this chip are no more than a few micrometres. Photo: SINTEF
“The technology can also be used to simulate such mechanisms as the so-called blood-brain barrier”, explains Skottvoll. “This is the body’s fail-safe mechanism that prevents unwanted substances from passing between the brain and our blood circulation system. Our brains are deliberately separated from our circulation system, which in the first instance is a good thing, but it can be a disadvantage in situations where we may want to medicate the brain”, she says.
It has traditionally been very difficult to study this function in humans, but organ-on-a-chip technology has now made this possible.
But what exactly is a stem cell?
As well as having a microstructure that mimics biological processes at cellular level, we also need a stem cell that can act as a ‘seed’ for the entire process. But what exactly is a stem cell?
According to the Norwegian Biotechnology Advisory Board, stem cells constitute the starting point for all the cells and tissues in our bodies. They are also responsible for tissue maintenance and the repair of damaged tissue. Stem cells have the unique ability to regenerate and divide over prolonged periods. They can also develop into more specialised cells by means of a process called cell differentiation. In this way, a stem cell can act as a ‘parent’ to everything from blood cells to muscle cells. However, a stem cell can never develop into an egg or sperm cell.
“Once we know what we want to do, it’s a bit like making sourdough”, says Skottvoll. “On day one, you complete the first step. On day two, you do something else, and then follows a lot of stirring and waiting until eventually you can add more ingredients. And so it goes on until we are satisfied that the cells have developed into an organoid. Sometimes, they will even develop into the same shape as a real organ”, she says.
Answers to many questions
Skottvoll became interested in organoid technologies at an early stage in her career. She had in fact started working with organ-on-a-chip technologies when she was a doctoral student at the University of Oslo, where she was able to observe exactly how much a ‘mini-liver’ resembles a real organ when exposed to a variety of drugs. (See the illustration earlier in this article.)
“What I found was that they behave in a very similar way to human livers”, she says. “However, they respond more slowly than real organs when it comes to breaking down drugs. This means in practice that experiments using such organoids may take time because they are not ‘fully” developed. We believe that the reason for this is that organoids have not yet been developed with a vascular system that can transport drugs internally”, says Skottvoll.
Can simulate anything
Thus, the physical basis of organ-on-a-chip technology is a microscopic structure consisting of membranes and a network of microfluidic channels. It is microstructures of this type that are now being manufactured at the SINTEF MiNaLab at Gaustadbekkdalen in Oslo.
“But just as important as producing organs ‘on-a-chip’, are the measurements we make of what is actually happening”, says Skottvoll. “For this reason, we’re also developing sensor technology that monitors automatically how the organoids are functioning on their chips”, she says.
Goodbye to animal testing and more rapid drug development
In the future, it may be that this technology will make animal testing redundant. We may also be able to cultivate organs instead of using donors, and to test drugs and therapies on ‘real’ human organs.
It is in fact possible even today to run advanced simulations of cell behaviour such as ‘in vitro’ embryo development – conducted entirely in a cell culture in a laboratory.
“Naturally, this is a field that raises a number of issues in terms of research ethics”, says Skottvoll. “Research into human brain organoids is a particularly hot topic at present”, she says.
However, there is no doubt that this is a technology that can be used to provide us with more effective methods of treatment, not least in the field of what is known as personalised medicine.
According to Skottvoll, it will change the way in which we select patient treatments.
“In the case of a patient with liver cancer, it will be possible to grow a patient specific ‘mini-liver’ and apply organ-on-a-chip technology to simulate how various drugs and therapies impact on the cancer and the patient’s liver”, she explains. “We will thus avoid having to test ineffective drugs on the patient, and the patient will avoid having to suffer any person-specific side-effects of the drugs”, says Skottvoll.
Click here to read more about organoids and the ethical debate surrounding their use.


