Researchers discover more about what causes atherosclerosis
The underlying cause of many cardiovascular diseases is inflammation of the artery walls. Now NTNU researchers have found that a specific neurotransmitter in the immune cells is a key factor when cholesterol accumulates in our blood vessels.
“We knew that part of the picture involved cholesterol inflammation, where the cholesterol was creating crystals that trigger the immune system. So we started exploring that,” says Nathalie Niyonzima. She is a researcher at CEMIR and at NTNU’s Department of Clinical and Molecular Medicine.
In a healthy person the immune cells break down cholesterol, and whatever the body does not need is transported out of the body through the urine. However, as cholesterol levels increase, immune cells are unable to maintain the proper balance, causing cholesterol crystals to form. These crystals create what is called a sterile inflammation, that is, an inflammation that is not caused by bacteria. This condition is called atherosclerosis.
Niyonzima and the rest of the team at CEMIR and NIH recently published the article “Mitochondrial C5aR1 activity in macrophages controls IL-1β production underlying sterile inflammation” in Science Immunology.
- You might also like: Cholesterol crystals play an active role in stroke, heart attacks
Chronic inflammation
Worldwide, cardiovascular disease causes 17.9 million deaths annually and was thus the leading cause of death in 2019.
The most common such illnesses are coronary heart disease (angina pectoris and heart attack) and cerebrovascular disease (stroke, stroke and cerebral haemorrhage).
The underlying cause of these conditions is atherosclerosis, a disorder that develops slowly and results in the formation of a chronic inflammation of the artery wall that over time can become life-threatening. Plaques consisting of dead immune cells, cell debris and fats build up along the walls of the arteries.
Overloading the arteries can then cause the blood vessels to become completely blocked.
“The problem is that when the immune cells find these cholesterol crystals, they don’t know what to do with them. They just know that something’s there that shouldn’t be,” says Niyonzima.
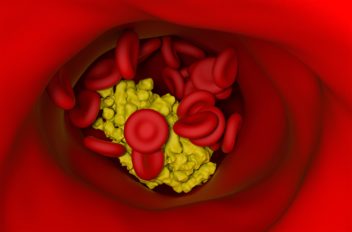
Plaques consisting of dead immune cells, cell debris and fats build up along the walls of the arteries. Too much debris can eventually completely block the blood vessels.
The immune cells in question are macrophages, cells that are found in our blood and which have the task of assaulting invaders like viruses and bacteria. The macrophages are the body’s first line of defence and “eat” these invaders.
“When the macrophages fail to break down the crystals, they become damaged and die. They can also break open so that the cell contents drain out, not a good thing. Things should preferably stay in their proper place,” Niyonzima says.
- You might also like: Staying fit can halve your risk of heart attack
Alarm goes off
Central to the work that has now been published is what happens inside the macrophage cells when they encounter these crystals.
When the immune cells attack the crystals, a communication system is activated inside the cell. From the cell wall, a signal is sent to a receptor on the surface of the mitochondria, the cells’ energy factories. The researchers in Trondheim have studied this reaction by adding cholesterol crystals to blood samples from healthy donors.
“This triggers the production of anti-inflammatory substances, at the same time as the energy production in the cells increases,” Niyonzima says.
The mitochondria start producing a specific cytokine (IL-1β). Cytokines are a type of protein that act like neurotransmitters and regulate immune responses.
“Our findings suggest that the receptor on the mitochondria acts like an alarm system that detects the cholesterol crystals and triggers the IL-1β production and inflammation in atherosclerosis,” says Niyonzima.
The researchers in Trondheim confirmed their findings by using mice that had a gene mutation for this particular receptor inside their immune cells. In these mice, the recipient of the neurotransmitter, the “on/off” button for the immune response, was thus “turned off.”
“We found that removing the gene reduced the size and degree of inflammation in atherosclerotic plaques. We also added inhibitors of the neurotransmitter to plaques from patients with atherosclerosis, and this significantly reduced the general inflammation in the plaques,” she says.
Commonalities with Alzheimer’s
In the same study, they also found that the genetic modification protected the mice from kidney failure.
Niyonzima explains that this internal communication system is an important factor in the biological process that leads to sterile inflammation as occurs in atherosclerosis. The possibility of using inhibitors for the neurotransmitter to treat the disease is another key element.
The results may also be important for the treatment of other serious diseases.
“This communication system works both internally inside the cells and externally, from one cell to another. What we show in this study is how the system uses a specific receptor on the mitochondria that starts up the whole ‘factory’ and that eventually leads to inflammation,” Niyonzima says.
“We see a bit of the same pattern in other diseases like gout and in the brains of Alzheimer patients. Overproduction of a protein creates aggregates that the immune system can’t handle,” she says.
Niyonzima emphasizes that there is still a long way to go before the results from her lab bench in Trondheim can be turned into a finished pill that can hinder the development of atherosclerosis in patients.
“This is basic research, not clinical research. We’re sitting with a large puzzle where a lot of pieces have to be put together for us to understand how everything going on inside the blood vessels is connected. But we think this is an important contribution. It’s definitely an important piece of the puzzle.
Reference: Nathalie Niyonzima et al: Mitochondrial C5aR1 activity in macrophages controls IL-1β production underlying sterile inflammation. Science Immunology.