NTNU researchers have found promising COVID-19 drugs
NTNU researchers are on track to find drug combinations that could help stop the coronavirus across the globe.
Hospital capacity has been pushed to the breaking point in many countries due to seriously ill coronavirus patients, and death rates continue to rise. Although COVID-19 vaccination is underway, the world will have to live with the coronavirus for a long time to come.
New combinations of existing medicines could be an important tool in preventing deaths and in helping patients infected with the coronavirus so that they become less seriously ill and recover faster. Researchers at NTNU have found several promising combinations of medicines.
Now these combinations will start to be tested on COVID-19 patients in clinical studies.
“If we had effective medication to treat patients as soon as they show symptoms or are diagnosed with infection, the course of the disease could be stopped with the right medicines,” says NTNU Professor Magnar Bjørås.
“This is how we could avoid both deaths and overloading hospitals with very sick coronavirus patients – for the good of the individual and of society,” Bjørås said. He is a professor and researcher at NTNU’s Department of Clinical and Molecular Medicine.
- You might also like: Not enough COVID-19 tests? No problem, we’ll make them!
Early treatment important
The treatment is most effective in early stages of the disease.
“The best strategy is to treat COVID-19 patients at an early stage before the coronavirus has the opportunity to spread into the respiratory tract and lungs and multiply and do a lot of damage,” says Bjørås.
“One reason infected patients begin to get seriously ill is because they’re having an inflammatory reaction in their lung cells. And out-of-control inflammation is often the reason why COVID-19 patients die,” he says.
Inflammation occurs 10 to 20 days after a person becomes infected.
“From the time a patient gets symptoms until they become seriously ill, you have a window where it’s easier and more effective to treat the patient than later in the course of the disease,” Bjørås says.
That’s why researchers are in favour of a front-line treatment to knock back the virus before it does too much damage – in other words, stopping the virus’ activity either outside the cell or inside the cell as quickly as possible.
- You might also like: Combination drug treatments for COVID-19 show promise in cell culture tests
Cultivating millions of lung cells
In March 2020, the research team, led by Professor Denis Kainov, began laboratory experiments in the hunt for COVID-19 treatment drugs. The team at NTNU also includes PhD candidates Aleksandr Ianevski and Erlend Ravlo, researcher Wei Wang and senior engineers Hilde Lysvand, as well as Magnar Bjørås, who has also been a key figure in the development of NTNU’s coronavirus test.
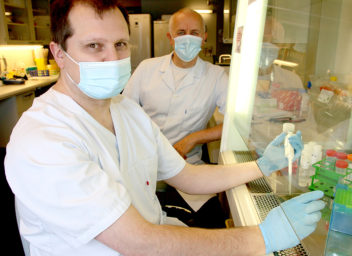
Professor Denis Kainov and Professor Magnar Bjørås in the laboratory, where they are testing different medicines on mini-lungs (organoids) that they have grown. Photo: Idun Haugan/NTNU
“Denis Kainov developed a model for virus infection in the lab. This is a model that has been used previously for other viruses, but Denis optimized it for SARS-CoV-2,” says Bjørås.
In the Department of Clinical and Molecular Medicine lab, researchers grow human cells that they use in their experiments. Since early last spring, the research group has grown lung cells for use in extensive experiments where they have been testing a number of antiviral drugs that may have an effect on the coronavirus.
The research project to find possible new treatment strategies also involves researchers from St. Olavs Hospital, Oslo University Hospital, the University of Tartu in Estonia and the University of Helsinki.
How the experiments work
Bjørås explains how the experiments work. “We extract cells from human lungs that we grow in the lab. Cultivation takes place in what are called well plates or culture flasks. Here the cells are fed and have oxygen and everything they need to multiply. The cells divide, enabling millions of cells to be produced in the lab,” he said.
First, the researchers infect the lung cells. The virus penetrates the cells in order to multiply and thus reduce functionality or kill the lung cells, like the coronavirus does in infected people. The only way the virus can multiply is by entering a human cell. If the virus doesn’t multiply inside the body, we won’t get sick.
Antiviral medication added
Then the researchers add broad-spectrum antiviral drugs to study whether this can help the cells survive the virus invasion. These antiviral drugs are also called inhibitors. They slow down the virus’ ability to replicate.
The medicines don’t remove viral infection, but they slow down its development and thus help the body’s immune system in fighting the virus.
The virus medicines that the researchers have tested are all approved for use and have been used for many years. What’s new is how the researchers are combining them to assess the benefits or synergy in how different drugs work simultaneously against the viral infection.
Another advantage of combining medicines is that you can reduce the dose of each, which may cause fewer side effects and provide more targeted treatment.
Gatekeepers prevent virus from entering
The researchers have tested drugs that have different effects. One type of medicine attacks the virus when it enters the body’s cells, so the drug acts as a gatekeeper to prevent the virus from entering the lung cells.
“If we imagine that a cell is a house with two doors: the gatekeeper medicine locks one door and thus prevents the virus from entering. But the other door has to remain slightly ajar for the cell to be provided with the necessary nutrients,” says Bjørås.
The crack in the second door can still enable the virus to sneak in.
Bodyguards deal with invaders
“Another type of medicine is therefore needed: a medicine that acts as a bodyguard and deals with the invaders. The bodyguard medicines destroy the virus’ RNA (genetic material) or prevent the virus from replicating.
If the virus is allowed to multiply inside the cell, it can eventually kill it and move on to new cells to destroy them.
“We’ve investigated a number of different medicines in combination. We’re seeing a very high survival rate of lung cells and that the virus doesn’t multiply but instead dies out,” says Bjørås.
“That leads us to believe that combination therapy is a good strategy for fighting the virus,” he said.
(Article continues under picture.)
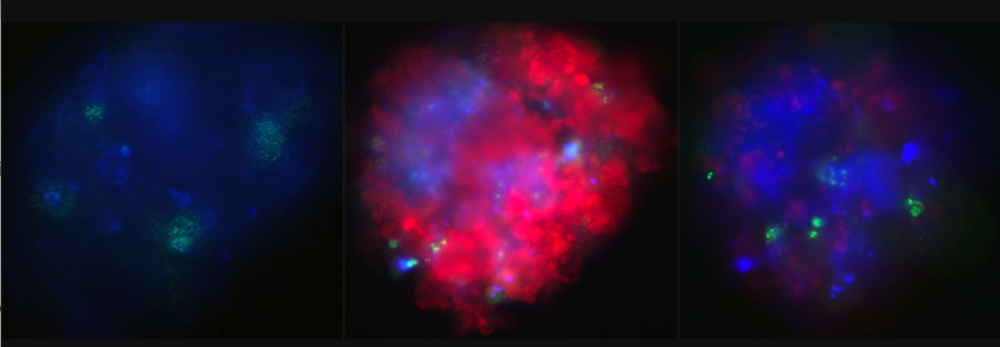
Microscope images of mini-lungs: To the left are healthy cells. Til venstre bilde av friske celler. The middle image shows cells infected by the coronavirus. The cells on the right have been given medicine so that they are healthy again. Image: NTNU
The researchers have combined more than 200 different medicines in all number of possible ways.
This spring, they have come up with 15 medicines that are interesting, and 5 combinations of these that are particularly promising.
In a recently published scientific article, the researchers conclude, “Overall, the development of combination therapies could have a global impact on improving the preparedness and protection of the population against new virus threats.” (Link to the article is at the bottom of this article).
Making mini-lungs to test combination medicines
This autumn and winter, the researchers have continued their work with more experiments, and have come up with several promising combinations that seem to work effectively against the coronavirus.

A researcher gives lung cells and mini-lungs (organoids)the nutrition they need to grow in petri dishes in the lab. Photo: Idun Haugan/NTNU
Researchers have also gone a step further by introducing anti-inflammatory drugs in combination with the antiviral inhibitors. Anti-inflammatory drugs work against the inflammation that coronavirus creates if the virus is allowed to multiply inside the body’s cells.
The researchers have also grown mini-lungs in the laboratory, which are more complex than the lung cells they used to begin with. The mini-lungs are used for further testing of the most promising combinations.
“The medicine combinations and the treatment are also proving to work well on the mini-lungs. This is a good sign,” says Bjørås.
Tested on humans in clinical trials
Some of the medicine combinations from the Kainov and Bjørås groups have already been tested on hamsters with promising results. The team is now working on starting clinical studies, which involve trialling the medicines on people. This will probably happen before the summer.
Individuals who participate in these clinical trials are voluntary participants. Clinical trials with testing on humans are most often initiated when the drugs have shown good results in lab experiments on animals, with only few or harmless side effects.
The first phase involves testing the combination drugs on relatively few people who aren’t necessarily infected with the coronavirus. This is to make sure that combining the medications doesn’t produce any side effects.
In phase two, the combination therapy is tested on COVID-19 patients and then on a larger group of patients, anywhere from a few hundred to several thousand.
International networks collaborating on clinical trials for COVID-19 treatment
The research group at NTNU is collaborating with research institutions and networks in the EU and the USA that are planning and carrying out clinical studies for new treatments of COVID-19 patients. Researchers at Oslo University Hospital and St. Olav’s Hospital are also part of these collaborative efforts.
“In these networks, several research groups and hospitals work together to find effective medicines for the virus,” says Bjørås.
These collaborative platforms are used to test existing drugs for new diseases, combinations of known drugs, or completely new active ingredients, where testing for any side effects is also crucial.
Studies on possible COVID-19 drugs are now taking place at a rapid pace in many laboratories around the world. Bjørås believes that the NTNU researchers and their partners have come a long way with promising results that are of interest for clinical trials.
More information:
https://www.mdpi.com/1999-4915/12/6/642
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7589631/
https://www.biorxiv.org/content/10.1101/2021.01.05.425331v2.full.pdf